Microbiome definition and role in human health
What is the microbiome?
Microorganisms have been here for billions of years, populating our world well before we appeared on the planet, and as humans we have evolved to live in close relationship with them1. Through co-evolution, the host not only tolerates but needs colonization by beneficial microorganisms for many aspects of immune development and function2. As a result, communities of microorganisms inhabit our body. These are principally bacteria but also viruses, fungi, archaea and bacteriophages.
The catalogue of all these microorganisms that live on, as well as in, the human body, together with their genetic complement, is called the microbiome. Microbiomes are present in different sites of the human body:

Skin

Mouth and nose

Vagina

Lung

Gut
The combinations of microbial taxa associated with the different surfaces of our body (microbiota) depend on the specific environment they live in and the host genetics3.
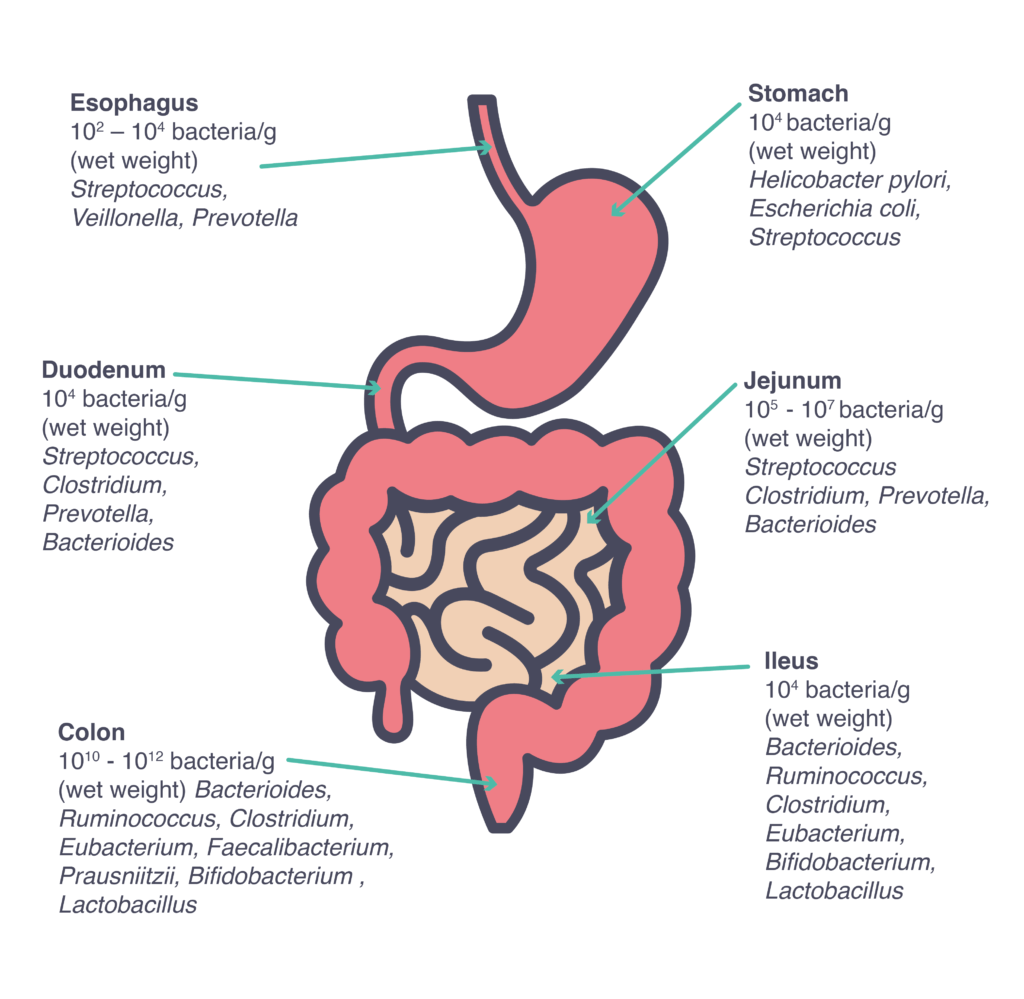
Although several different microbiomes can be found in a human body, the vast majority of our microbiota is located in the gastrointestinal tract3, considered as one of the most densely populated ecosystems on our planet, with about 10 billion microorganisms, with numbers and species varying along the tract4.



Recently, the gut microbiota has been a trending topic.5 The majority of research is currently focused on the gut microbiota6, with over 10,000 research papers on the topic published in 2021 alone. Over the last decade, new technologies have facilitated large-scale analysis of the genetic and metabolic profile of microbial communities6, increasing our knowledge and enabling a deeper understanding of the role of the gut microbiota in health and disease.5
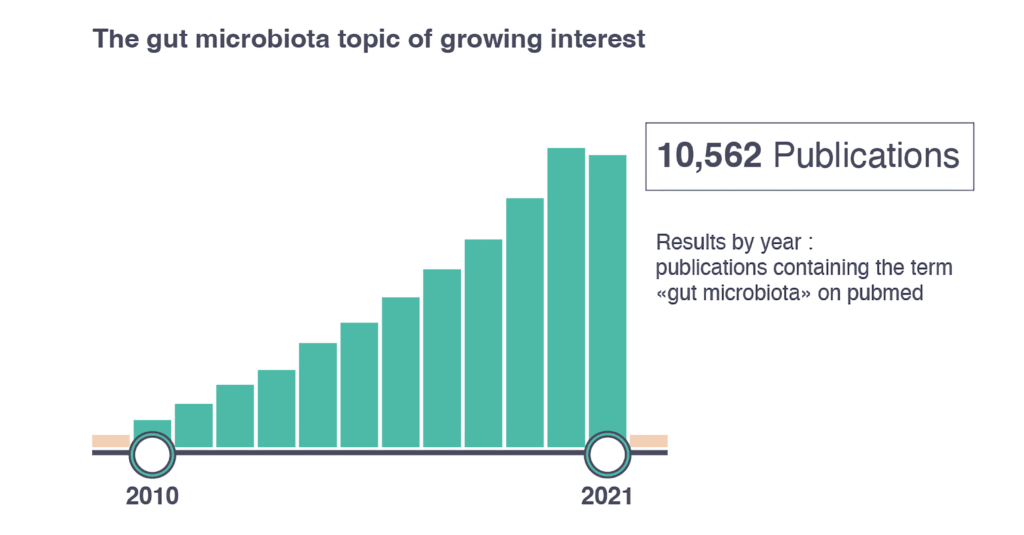
This evidence has contributed to new perspectives and new study techniques have highlighted mutual, symbiotic benefits between humans and the microbiome of the digestive tract.7
References :
1 Hill C et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506-14. d 2 Lin L and Zhang BMC Immunology (2017) 18:2 3 Ursell L, et al. Defining the human microbiome. Nutr Rev. 2012;70:S38-S44. 4 Coudeyras S and Forestier C. Microbiota and probiotics: effects on human health. Can J Microbiol 2010;56:611-50. 5 Mills S, Stanton C, Lane JA, Smith GJ, Ross RP. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients. 2019 Apr 24;11(4):923. 6 Marchesi JR, Adams DH, Fava F, et al. Gut 2016;65:330–339. 7 Singh Y, et al. Emerging importance of holobionts in evolution and in probiotics. Gut Pathogens 2013;5;12.
The role of gut microbiome in human health
The gut microbiome is integral to the health of its host: while protecting against pathogens through colonization resistance, it interacts with human cells, directly or indirectly through microbially-produced bioactive molecules, regulating numerous biological pathways.1 When operating optimally, the microbiome plays an important role in human health by supporting protective, metabolic, and immune functions.2



The gut microbiota can weigh up to 2Kg.
Microorganisms impacting the organism
The relationship between the intestinal microbiota and epithelial cells supports systemic immunity, neuro-endocrine function, intestinal and extra-intestinal health from infancy to adulthood.2,4,5
The microorganisms that colonize the digestive tract are responsible for numerous essential functions within the human body, including:
Digestion:
- Assisting with digestion and metabolism5
- Providing essential nutrients, vitamins and bioactive metabolites, but also neurochemicals that can influence the peripheral enteric and central nervous systems1
Immunostimulation:
- Promoting and regulating the immune system3.
Protection:
- Maintaining the gut barrier integrity
- Preventing pathogen colonization, directly or indirectly1
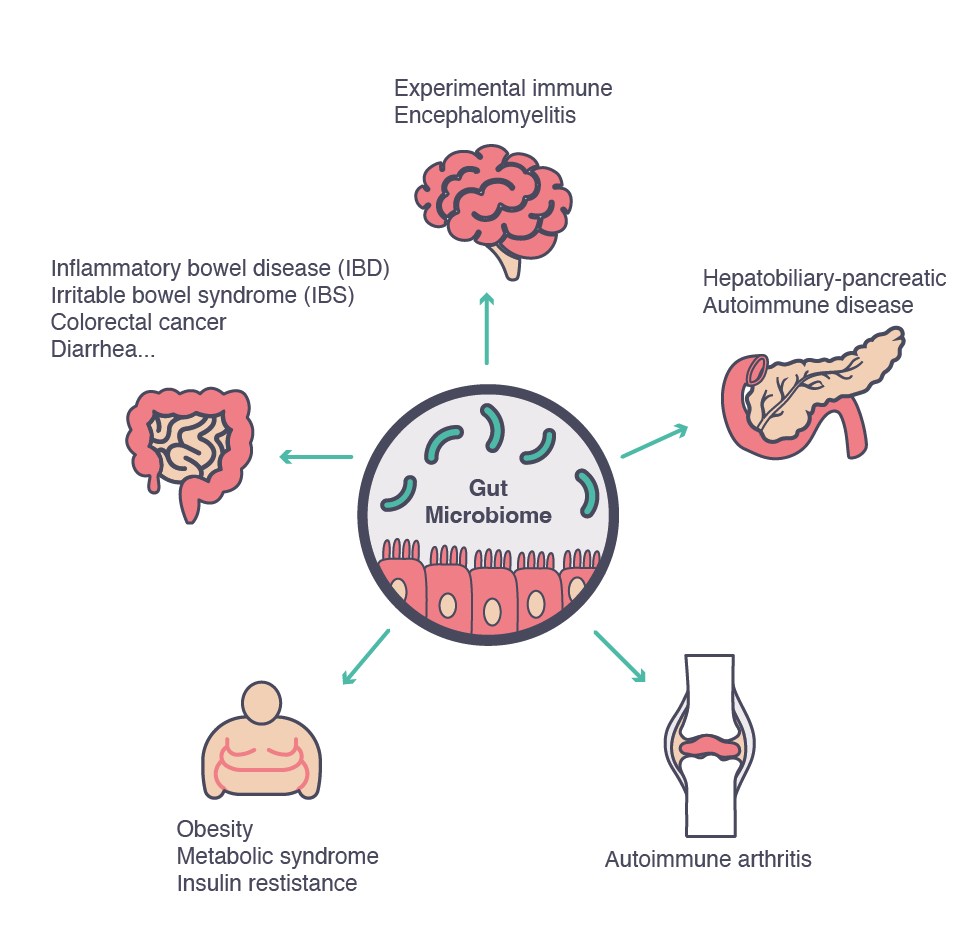
While healthy microbial communities can sustain our well-being, a dysfunctional (dysbiotic) microbiota is implicated in many gastro-intestinal diseases, including diarrhea and chronic digestive diseases.6 Furthermore, a microbiota that has deviated from the ‘healthy’ status in terms of diversity and functionality can also affect other organs and systems and has been implicated in non-GI diseases, such as asthma, obesity, diabetes and autoimmune conditions, all characterized by the over-responsiveness of the immune system, which in turn leads to an increasingly pro-inflammatory status.3
References :
1 Mills S, Stanton C, Lane JA, Smith GJ, Ross RP. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients. 2019 Apr 24;11(4):923. 2 Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67(9):546-50. 3 Ursell L, et al. Defining the human microbiome. Nutr Rev. 2012;70:S38-S44. 4 Neu J, Douglas-Escobar M, Lopez M. Microbes and the developing gastrointestinal tract. Nutr Clin Pract. 2007;22(2):174-82. 5 Huffnagle GB. GI Microbiota and Regulation of the Immune System. New York: Springer-Verlag New York; 2008. 149 p. 6 Goldszmid RS and Trinchieri G. The price of immunity. Nat Immunol 2012;13:932–38.
The importance of a balanced microbiome
The gut microbiota is a highly complex community which develops immediately after birth, evolves through to old age1 and varies significantly from host to host.2
In addition to the stable set of gut colonizers that are always present, known as the core gut microbiota and genes or core microbiome, everyone has their own specific set of gut microorganisms.1
Under normal conditions, the healthy adult microbiota is generally characterized by a rich diversity of species that remains stable over long periods of time, however, it can be influenced by a number of factors.2 Beyond age and host genetics3, the microbiota is influenced by geographical location (diet could be an important contributing factor), antibiotic usage, non-antibiotic drugs, illness, injury, hormonal changes2, a sedentary lifestyle1 and stress.3

In this symbiotic relationship, the human body receives several benefits: we provide a stable, nutrient-rich environment, while the microorganisms are sustaining our health with many important functions, including stimulation of the immune system, improved digestion and absorption of food, reduced growth of pathogenic flora, and maintenance of intestinal barrier integrity.3
These beneficial effects can be observed not only locally, but also in distant organs, due to systemic distribution of substances and cells produced in the intestine.3 The benefits are dependent on the quantity and quality of the microbiota and its metabolic potential.2 These positive mechanisms can be disrupted due to an altered microbial composition, known as dysbiosis.5
Science continues to uncover and explain the composition and functionality of life stage-specific gut microbiota and deviations from what is considered “normal” or “healthy”.2 With increasingly sophisticated methods to profile and characterize complex ecosystems, the involvement of the microbiota in a large number of intestinal and extra-intestinal diseases has become more apparent.5
Dysbiosis
Given the close symbiotic relationship existing between the gut microbiota and the host, it is not surprising to observe a divergence from the normal microbiota composition in a range of disease states, from chronic gastrointestinal diseases to neurodevelopmental disorders.5
The evidence of an alteration of the gastrointestinal microbiota contributing to the development of pathological conditions is increasing.1 Many studies have shown that an imbalance in the intestinal microbiota can lead to the development of conditions such as allergic or autoimmune diseases (e.g., inflammatory bowel disease and type 1 diabetes, among others).
Furthermore, comparison of the diversity of microbial populations between different individuals has helped identify their association with different pathological conditions. Many such studies describe associations between the presence or absence of a range of microbial species and a particular disease, further supporting the hypothesis linking dysbiosis and the etiology of various disease states.7
Impact of the gut microbial changes in various non-communicable diseases1
| Type | Disease | Predominant genus/phylum | Decreased genus/phylum | Mechanism/role in developpemnt of disease |
|---|---|---|---|---|
| Metabolic | Obesity | Firmicutes | Bacteroides, Akkermansia muciniphila | Inflammation caused by LPS |
| Metabolic | Type 2 diabetes mellitus | Bacteroidetes and Proteobacteria, Clostridium | Firmicutes, Lactobacillus, F. prausnitzii, Roseburia intestinalis, Roseburia inulinivorans and E. rectale | Inflammation caused by LPS |
| Vascular | Atherosclerosis | Clostridia, Proteus, Shigella and Aerobacter | Metabolism of trimethylamine to Trimethylamine N-oxyde | |
| Infection | Clostridioides difficile-associated diarrhea | Clostridioides difficile, minor constituents of the gut | Bacteroidetes, Firmicutes | Alteration of normal gut microbiota, overgrowth of Clostridioides difficile in the gut |
| Malignancy | Colon cancer | Helicobacter pylori, Enterococcus faecalis | E. rectale and F. prausnitzii | Unbalanced pro-inflammatory signaling by colitogenic bacteria, direct cytotoxic effect, metabolism of certain compounds producing carcinogenic by-products |
| Autoimmune disorder | Rheumatoid arthrisis | Prevotella copri | Bifidobacterium, Bacteroide – Porphyromonas – Prevotella, Bacteroides fragilis subgroup and E. rectale – Clostridium – Coccoides group | Induction of differenciation of CD4+ cells to Th17 cells and production of autoantibodies |
| Autoimmune disorder | Celiac disease | Bacteroides, Prevotella and Escherichia coli | Bifidobacterium, Lactobacilius, F. prausnitzii | Induction of pro-inflammatory signals through activation of enterocytes or classical APCs |
| Inflammation | Inflammatory bowel disease- Ulcerative colitis | Facultative anaerobes | R. hominis and F. prausnitzii | Aberrant host response towards its gut microbiota |
| Inflammation | Crohn’s disease | Faecalibacterium prausnitzii, Bacteroides, Eubacterium | Decrease in the overall diversity of gut microbiota | Aberrant host response towards its gut microbiota |

As science continues to delineate the composition and functionality of life stage-specific gut microbiota and deviations from what is considered “normal” or “healthy”, opportunities arise for dietary and therapeutic interventions which can beneficially modulate the microbiota and result in translational benefits to host physiology and overall health.10
References :
1 Pushpanathan P, Mathew GS, Selvarajan S, Seshadri KG, Srikanth P. Gut microbiota and its mysteries. Indian J Med Microbiol. 2019 Apr-Jun;37(2):268-277. 2 Mills S, Stanton C, Lane JA, Smith GJ, Ross RP. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients. 2019 Apr 24;11(4):923. 3 Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients. 2020 Jul 23;12(8):2189. 4 Lin L and Zhang BMC Immunology (2017) 18:2 5 Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017 May 16;474(11):1823-1836. 6 Goldszmid RS and Trinchieri G. The price of immunity. Nat Immunol 2012;13:932–38. 7 Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020 Dec;113(12):2019-2040. 8 Salminen, S., Collado, M.C., Endo, A. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol (2021). 9 Vijay, A., Valdes, A.M. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr (2021). 10 Mills S, et al. Precision Nutrition and the Microbiome Part II: Potential Opportunities and Pathways to Commercialisation. Nutrients. 2019 Jun 27;11(7):1468.
